Background: Myelofibrosis (MF) is a rare hematologic malignancy for which treatment has evolved considerably in recent years, with 3 JAK inhibitors now approved in the United States to manage this disease. With numerous therapeutics in phase III trials, the complexity of treatment selection is likely to increase in the coming years. To assist hematology/oncology healthcare professionals (HCPs), we developed an online decision support tool designed to provide case-specific treatment recommendations from 5 MF experts. Here, we report an analysis of cases entered into the tool by HCPs, comparing their planned treatment with expert recommendations to determine differences in clinical practice and areas of educational need.
Methods: In February 2023, 5 MF experts provided evidence-based treatment recommendations for 45 distinct MF case scenarios that were defined by multiple key factors, including the presence of MF symptoms, splenomegaly, or anemia; platelet count; prognostic risk group; and previous JAK inhibitor therapy. To use the tool, HCPs entered their patients' characteristics and their intended treatment plan; recommendations from each of the 5 experts based on those same characteristics were then provided. In this analysis, the intended treatment plans of HCPs were compared with expert recommendations to determine areas of discordance and agreement.
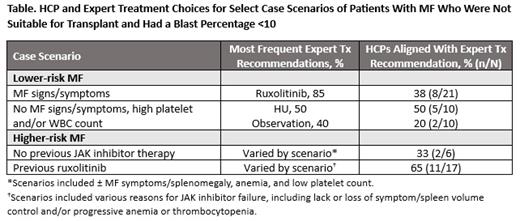
Results: In May/June 2023, 91 cases were entered into the tool (84% of cases by physicians); 72% of respondents reported treating 1-4 patients with MF per month (n = 32), and 61% sought recommendations for a specific patient in their practice (n = 28). A lack of concordance between HCP and expert treatment choices was observed for many case scenarios (Table). For example, for case patients with lower prognostic risk and MF signs/symptoms, experts most frequently recommended ruxolitinib (85%) in contrast to HCPs, who recommended ruxolitinib for 38% of these cases. Similarly, discordance was noted for several case scenarios of patients with higher-risk MF who were not suitable for transplant and required a treatment change following initial therapy with ruxolitinib. Of note, subtle variance was observed among experts for specific second-line case scenarios, including when to add on to current therapy vs switching to a new JAK inhibitor, which may reflect the burgeoning area of clinical practice in defining JAK inhibitor failure and determining subsequent second-line treatment paradigms with several JAK inhibitors now available. Of the 23 HCP cases seeking recommendations for patients with higher prognostic risk, 74% sought recommendations in the post-ruxolitinib setting. Overall, after reviewing expert recommendations, 81% of HCP respondents indicated that they would change their treatment approach or that the recommendations confirmed their treatment strategy (n = 32).
Conclusions: Analysis of data from an online decision support tool indicates differences among experts and HCPs in contemporary management of patients with MF, and data suggest that a decision support tool can affect HCP treatment decisions in a rapidly evolving therapeutic landscape, potentially improving patient care. In-depth analyses with additional HCP cases will be presented.
Disclosures
Bose:GSK, Novartis, Karyopharm, AbbVie, Pharma Essentia, Jubilant, Morphic: Honoraria; Kartos, Telios, Ionis, Disc, Janssen, Geron: Research Funding; Incyte, BMS, CTI, Morphosys, Blueprint, Cogent, Sumitomo: Honoraria, Research Funding. Mascarenhas:GSK: Honoraria; Incyte, Novartis, Roche, Geron, GSK, Celgene/BMS, Kartos, AbbVie, Karyopharm, PharmaEssentia, Galecto, Imago, Sierra Oncology, Pfizer, MorphoSys, CTI Bio: Consultancy; Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Janssen, Kartos Therapeutics, Merck, Novartis, PharmaEssentia, Roche; Participated in consulting or advisory committees - AbbVie, Bristol Myers Squibb, Celgene, Constellation Pharmac: Research Funding; Bristol Myers Squibb, Celgene, Constellation Pharmaceuticals/MorphoSys, CTI BioPharma, Galecto, Geron, GSK, Incyte Corporation, Karyopharm Therapeutics, Novartis, PharmaEssentia, Prelude Therapeutics, Pfizer, Merck, Roche, AbbVie, Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Novartis, Janssen, Kartos Therapeutics, Merck, PharmaEssentia, Roche: Research Funding; AbbVie, CTI BioPharma Corp, a Sobi company, Geron, GlaxoSmithKline, Imago, Incyte, Kartos, Kayropharm, MorphoSys, Novartis, Pfizer, PharmaEssentia, Sierra: Consultancy. Rampal:Dainippon: Consultancy; Karyopharm: Consultancy; CTI BioPharma Corp: Consultancy; Incyte: Consultancy; GSK-Sierra: Consultancy; Zentalis: Consultancy; Servier: Consultancy; Kartos: Consultancy; Morphosys/Constellation: Consultancy; Sumitomo: Consultancy; Galecto: Consultancy; Pharmaessentia: Consultancy; Celgene-BMS: Consultancy; Incyte: Research Funding; Zentalis: Research Funding; Stemline: Research Funding; Constellation: Research Funding; Ryvu: Research Funding. Mesa:Novartis: Consultancy; Sierra Onc: Consultancy; LaJolla Pharma: Consultancy; Constellation: Consultancy, Research Funding; Celgene: Research Funding; Incyte: Research Funding; Abbvie: Research Funding; Samus: Research Funding; Genetech: Research Funding; Promedior: Research Funding; CTI BioPharma., a Sobi Company: Research Funding; Mays Cancer Center: Research Funding; NCI: Research Funding.